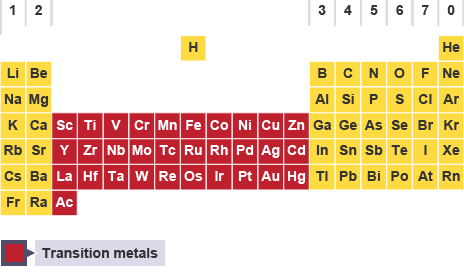
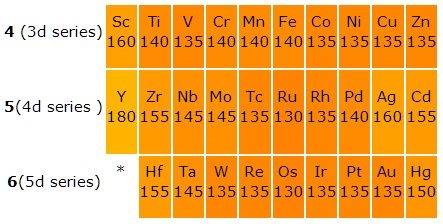
d and f-Block Elements
What are f block elements?
f –block elements are also called inner transition elements. In these the last electron enters penultimate i.e. (n – 2)f f orbital. The differentiating electron in transition elements may enter either 4f or 5f orbitals based upon which they are differentiated into lanthanides and actinides.
Lanthanides: In lanthanides the differentiating electron enters 4f orbital. These are cerium to lutetium. The name lanthanides is because they come immediately after lanthanum.
Actinides: In actinides the differentiating electron enters 5f orbitals. These are thorium to lawrencium. These elements come immediately after actinium.
Electronic configuration: General electronic configuration of f – block elements is (n–2)f1–14(n–1)d0–1ns2
- Lanthanides: [Xe]4f1–145d0–16s2
- Actinides: [Rn]5f1–146d0–17s2
Source
What are f block elements?
f –block elements are also called inner transition elements. In these the last electron enters penultimate i.e. (n – 2)f f orbital. The differentiating electron in transition elements may enter either 4f or 5f orbitals based upon which they are differentiated into lanthanides and actinides.
Lanthanides: In lanthanides the differentiating electron enters 4f orbital. These are cerium to lutetium. The name lanthanides is because they come immediately after lanthanum.
Actinides: In actinides the differentiating electron enters 5f orbitals. These are thorium to lawrencium. These elements come immediately after actinium.
Electronic configuration: General electronic configuration of f – block elements is (n–2)f1–14(n–1)d0–1ns2
- Lanthanides: [Xe]4f1–145d0–16s2
- Actinides: [Rn]5f1–146d0–17s2
sourcehttps://www.askiitians.com/iit-jee-d-and-f-block-elements/


Tambahkan Komentar Sembunyikan